Frequently Asked Questions
Validation of Your Medical Imaging
All owners of DSC phantoms: Please read our Special Notice regarding the use of alcohol and solvents in DSC phantoms.
Data Spectrum advises owners of DSC Phantoms to NEVER use alcohol or solvents in DSC Phantoms. This notice supercedes information found in manuals and elsewhere that was printed prior to April 2006.
Notice: Before continuing on, please read the following disclaimer:
Data Spectrum Corporation (DSC) does not warrant that the information provided in the Frequently Asked Questions (FAQ) section is complete, factual or correct, or will meet the requirements of the user, or be appropriate for any specific application. In no event will DSC be liable for any damages, including, but not limited to, lost profits, lost savings, or other incidental or consequential damages arising out of the use of DSC products or for the use of any information found on this Web site, including, but not limited to, the FAQ section, even if DSC has been advised of the possibility of such damages, or for any claim by any other party.
Frequently Asked Questions
What can I measure or do with DSC's phantoms?
- Simulate in vivo radiopharmaceutical distribution in an emission computed tomography (ECT) slice (SPECT or PET)
- Evaluation of data acquisition and reconstruction methods for brain ECT studies
- Research
- System single-slice volume sensitivity
- System total volume sensitivity
- Effect of regional variations in intrinsic system response using uniform cylindrical portion
- Accuracy of attenuation compensation algorithms
- Variation in spatial resolution within the field of view using the multi-sized rod insert
- Lesion detectability using the multi-sized spheres
- Effect of finite spatial resolution and Compton scattering on image quality
- Image contrast, %-RMS noise and signal-to-noise (SN) measurements
- Line spread measurements in air and in water
How do I know which phantom (or size rods/spheres/etc.) to buy?
For routine quality control purposes, many institutions select the Standard SPECT Phantom (Model ECT/STD/P). This is an all-around, general purpose, medium performance model. Its cold rod insert has rods from 6.4 to 16.0 mm and the spheres are 12.7 to 38 mm.
For higher resolution, we offer the Deluxe Phantom (Model ECT/DLX/P) and for even higher resolution, is the Ultra Deluxe Phantom (Model ECT/U-DLX/P).
For legacy systems or those with generally poor performance, we offer the Benchmark Phantom (Model ECT/BEN/P). The cold rods in its insert range from 9.5 to 25.4 mm and the 6 spheres are 12.7 to 38 mm
Please note that when you purchase a “QC” phantom of the circular cylinder type, you receive a cylinder with cover plate and the insert of your choice. If you wish to add to your imaging options, you can purchase only the inserts (rods or spheres) and use your existing cylinder.
Note that some of our other phantoms will have a single insert that is available. Please see our Products page see the full line of phantoms and options for each one.
The “QC phantom” line is only part of our offerings. Some phantoms are designed for evaluating neurological studies (brain phantom models include 2-D, 2-D multi-compartment and 3-D versions), torso phantoms, elliptical QC phantoms, a NEMA phantom and cardiac phantoms. We also supply a wide array of inserts and accessories, and a series of phantoms and inserts for super-high resolution “small animal” scanners.
So, please look through our catalog to find the phantom that you think best suits your needs. Of course, if you want help deciding or just have questions in general, please contact us. If you need additional alterations to an existing phantom to fit your needs or require a completely different imaging tool, we are open to working with you to create a custom piece.
Why Do I Need a Phantom?
Generally speaking, the most simple, straightforward way to know what resolution, image contrast, slice thickness, etc. your imaging system is capable of is to scan a phantom. In the phantom, you know ahead of time the true size of an object (or its contrast or whatever) that you are interested in measuring.
You cannot determine these parameters very easily in a patient, and even if you did, you probably won’t have the same patient back with exactly the same “configuration” month after month when you are performing Quality Control procedures … and you can’t fill arbitrary locations in patients with just any arbitrary amount of radioactivity.
With a phantom, you can:
- have a standardized test procedure
- compare your results from month to month
- compare your results to your other scanners, or to those at other institutions
- decide on clinical protocols for acquisition and processing ahead of time
- practice patient setup and image processing techniques
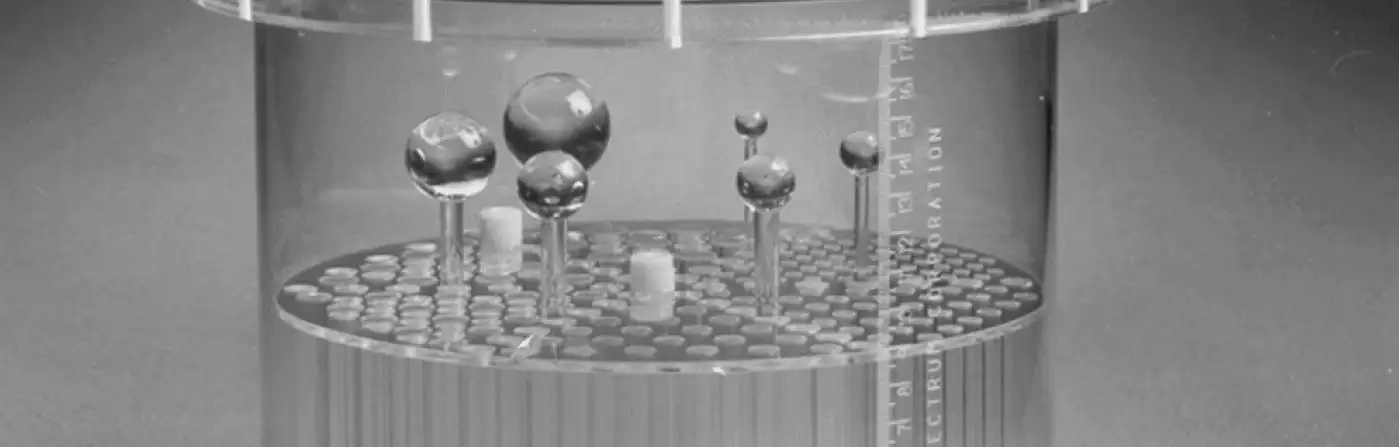
In what locations should I position spheres in the SPECT or PET QC phantoms?
Please see the accompanying figure for typical sphere locations. Note that between each sector of cold rods there is a hole designated for the sphere stems to pass through. The holes for the sphere stems are located half way between the center of the phantom and the phantom wall. In the figure-right below, the red arrows point to the cold area occupied by the sphere stems in a reconstructed SPECT image. The order in which the spheres are arranged (largest to smallest, clock-wise versus counterclockwise, etc.) is not important. It is also not necessarily important that the largest sphere be located next to the largest sector of rods. For phantoms other than the one shown, please refer to photos or diagrams in the manual shipped with your phantom, or see the brochures for it on this web site.
What kind of lubricant should be used on O-rings and where can I buy it?
We recommend using only silicone grease on O-rings. You must not use any petroleum containing product, which includes petroleum jelly (sometimes called “petrolatum”). A commercial product is Vaseline ®. We repeat: Do not use Vaseline ®, petrolatum or petroleum jelly on O-rings, for it will damage them.
One acceptable product for use on O-rings is “Silicone Lubricant” in the catalog at http://www.tryphon.it/catalogo.htm. The catalog description says “Special silicone lubricant with PTFE”, and it is fairly inexpensive.Another acceptable product for use on O-rings is Gunk Plumber’s Silicone Grease. The Gunk part number is GR2V. You can find more information about this product at http://www.gunk.ca/prodsht-en/gr2.pdf (link will open in a new window). We found Gunk Plumber’s Silicone Grease in local hardware stores and the plumbing department of home improvement companies (such as Lowes and Home Depot). It may also be available at plumbing supply companies and auto part dealers.
How do I receive the correct manual?
Data Spectrum Corporation provides each customer with a SPECT Phantom User’s Manual, which can be applied towards the general use and maintenance of both PET and Nuclear Medicine Phantoms approved by the ACR.The phantom consists of our Flangeless ECT Jaszczak Phantom, which includes a cold rod insert and a set of six solid spheres. The PET lid (sometimes referred to as a faceplate) was designed by Dr. Peter Esser who is associated with the American College of Radiology. The lid was designed for sites receiving accreditation from the ACR. The ACR has specific protocols for this process. You can find the ACR protocols by going to the ACR web site (www.acr.org) click on accreditation, Nuclear Medicine and PET, Testing and QC Forms, PET Phantom Instructions. The phantom is pre-assembled prior to packaging with the exception of the cold spheres, please note these are not used in the PET accreditation process.
What is the procedure for placement of the solid spheres?
The ECT phantoms are pre-assembled prior to packaging with the exception of the cold spheres. The set of spheres (6) are located within the cylinder, the spheres are mounted in the cylinder by gently screwing the bottom of the spheres into threads located on the bottom of the cylinder. Inside the cylinder when looking at the cold rod insert you will see the top plate and six pie shaped sections of cold rods. Between each section is a row of vacant holes. The spheres are inserted into the third vacant hole, which should line up with the threads on the bottom of the cylinder. For most users the largest sphere is set counter-clock wise from largest pie section. Gently screw the remainder of the spheres in the threads (going counter-clock wise) with the next size down to the smallest spheres next to each pie section.
How do I eliminate air bubbles in the Mini, Micro and Ultra Micro line of phantoms?
With the Mini and especially with the Micro and Ultra Micro line of phantoms, it can be difficult to always replace all the air in the phantom with liquid. Bubbles large enough to be visible to the unaided eye will likely be large in comparison with structures within the phantom. A reason for this difficulty is the surface tension of water makes it hard to flow easily into and then through small passages. Small (less than 1-2 mm) bubbles tend to stick to surfaces inside the phantom much more so than do larger bubbles. For any Data Spectrum Phantom, NEVER use any kind of alcohol or solvent to fill them. Use of alcohol or solvents of any kind is very likely to damage your methyl methacrylate (MMA) or polymethyl methacrylate (PMMA) phantom. Because the gaps between the plates (of the Defrise phantoms) or holes (in the Hotspot or cold rods in other phantoms) phantoms are small, you may encounter some small bubbles that adhere to the inserts and/or cylinder surfaces. You might find it helps to use a thinner or wetting agent (surfactant) of some kind that will reduce the tendency of small bubbles to adhere to the wall and gaps. A few drops of common dish washing detergent (that is, the kind that is used with automatic dishwashing machines) may be used to minimize this effect. For example, a solution of 1 mL dish washing detergent, (such as Cascade gel) added to 200 mL water may be tried. Also you may try 2 or 3 drops of liquid dish soap such as Ivory or Joy) added to 100 mL of water. This latter solution may produce too many bubbles, if the solution is stirred too vigorously. Be careful not to mix the solution so vigorously that soap bubbles form. Some hand soaps contain a surfactant and a few drops of these kinds of soap, added to 100 mL of water may also be tried. Avoid alcohols, solvents, and even any soaps or detergents that may contain alcohol or solvents of any kind. A trick to help determine where liquid has gone to in your phantom is to add a visibly high concentration of food coloring. Red food coloring works well. Be certain with some of the DSC phantoms to fill the phantom with it in the proper orientation. Also, some older style phantoms must be filled with a syringe under the phantom injecting upward, rather than downward into the phantom. Your User’s Manual for your particular phantom will show and explain to you exactly how to fill your phantom. Again, older manuals might state that it is alright to use alcohol in phantoms, but this is no longer the case.
How can I accurately simulate bone density in a fillable phantom (or insert)?
If you want to more accurately simulate bone, Data Spectrum sells a fillable spine that is a replacement for the Teflon one. A few years ago, a solution was proposed that was very similar to cranial bone, as far as attenuation characteristics are concerned. It consists of:100 gr of K2HPO4 (dipotassium hydrogen phosphate) dissolved in 67gm of H2O.Reference: De Dreuille, O., V. Strijckmans, et al. (1997). “Bone equivalent liquid solution to assess accuracy of transmission measurements in SPECT and PET.” IEEE Transactions on Nuclear Science 44(3): 1186-1190.We have found that it is best to use a magnetic stirrer and to heat the water/salt mixture to facilitate dissolving the salt. Once dissolved, the salt will remain in solution at room temperature.It was shown in the reference above that the attenuation properties of this solution is nearly equivalent to skeletal cranium bone over a range of 50 to 600 keV. This was also tested at the site of one of DSC’s customers and it worked very well from 140 keV up to 511 keV in their situation. The density of the solution is 1.68, while the density (according to the above reference.) of bone is 1.61. By weight, the composition of the solution is: 26%(K), 10%(P), 56%(O), 8%(H), while bone is: 17.6%(Ca), 8.1%(P), 43.5%(O), 5%(H), 21.2%(C), 4%(N). K (Z = 39) is used in place of Ca (Z = 40).
What is the density of the fillable lungs in certain body phantoms?
The fillable lung portions of certain phantom are typically filled with styrofoam beads and water. The degree to which the beads are packed into each lung will determine the density of each lung. Packing less beads in a lung means that more water can be packed in the lung, which means that the relative density of the lung will be higher. (“Fewer beads” means “more water”, and water being more dense than the beads means the overall density is higher). Here is a method for determining the fractional (beads/water) water density:a) Remove all beads (This can be messy). A small hand-held vacuum cleaner with a new (clean) filter bag installed to remove the beads can prove useful for this task, and then measure the lung volumes.b) Refill the lungs with dry beads.c) Add water and record the volume of water added to each lung. The fractional density (relative to pure water) is (neglecting the density of the Styrofoam(TM) beads) equal to about:(Volume of water added) divided by (total lung volume) One would then multiply the attenuation coefficient of water by this fraction. A more accurate estimate can be obtained by determining the attenuation coefficient for the Styrofoam(TM) beads and then estimating the fraction of lung volume that is occupied by the beads. Note that the attenuation coefficient for Styrofoam is approximately 0., nearly equal to air. The fractional bead volume is equal to:((the total lung volume) minus (the volume of water that was added) divided by the total lung volume). This fractional bead volume is multiplied by the bead attenuation coefficient at, for example, 511 keV and added the to the fractional water density attenuation coefficient at 511 keV to obtain the total 511 keV attenuation coefficient. This approach has proved successful for 140 keV photons by some some of our customers. Typically, F is about 0.3 to 0.4, depending on how tightly one packs the Styrofoam beads in to the lungs.You could perform a simple “narrow beam” transmission measurement using a 511 keV (or 140 keV, or whatever desired) source and a known thickness of the bead/water mixture:I/IZero = exp(-mu*thickness). All you have to do is measure I and IZero and solve for the linear attenuation coefficient “mu”.I is the measured flux (counts/sec) with the bead/mixture of known thickness placed between the “narrow beam” source and the scintillation detector, and IZero is the flux without the bead/water mixture. Of course, you should correct for room background, if necessary.A flat uniform thickness of the bead/water mixture would be best for this experiment. We suspect it would need to be relatively thick because of the small mu value at 511 keV, so you can determine a more accurate value. Obtaining a “narrow” beam source may be a bit of a challenge for 511 keV photons, but a few lead bricks strategically placed may work, or you may need to drill a hole in a few bricks and stack them on top of each other. It is much easier with Tc-99m. You may be able measure it at 140 keV and extrapolate to 511 keV, but we have no data to indicate how feasible/accurate this is. To summarize: The attenuation coefficient at some given keV (e.g., 511) is very nearly equal to a fractional amount of water at that keV. This is because the lungs consist of Styrofoam-like beads that displace the water. Here is how to calculate the fraction “F”:
1. Measure lung volume with no Styrofoam beads in it [V(total)]
2. Measure Lung volume with Styrofoam beads installed in lung [V(lung)]
3. The fraction F is simply the fractional amount of water that can be used in the presence of the beads and is equal to density of the lungs when it is multiplied by the density of water (1 gm/cm3):F = V(lung)/V(total) gm/cm3To repeat, typically, F is about 0.3 to 0.4, depending on how tightly one packs the Styrofoam beads in to the lungs.
What is the density of the Teflon spine in certain body phantoms?
Our solid spine insert is made of “high density” “virgin” Teflon. Narrow beam measurements of virgin Teflon at 140 keV yielded a mu (cm-1) value of 0.2853. Just FYI, if you should have a special order solid spine insert made of “mechanical” Teflon, this material has a measured mu (cm-1) at 140 keV of 0.2892. At 140 keV, both materials have essentially the same mu value.Other methods of determining density:A. If you have access to a calibrated TCT scanner, you can of course perform a scan of the material in question and get the “CT number” of the material from the images via regions of interest. You may have to include in this or other scans materials of known density, such as water, iodinated contrast material, etc., and then extrapolate CT numbers back to mu values for your particular object/material.B. You can perform your own determination of mu value. Using a narrow, highly collimated radiation beam from your specific radionuclide or X-ray source, measure the beam intensity with and without the virgin Teflon in between the radiation source and the detector. Example with sample variable names, where:
thick = object thickness in cm
ref = reference counts (no material between detector and radiation source)
obj = object counts (material is between detector and radiation source)
mu = mu value Calculations:
a = obj/ref
b = natural-log (a)
mu = b/thickSuppose that: obj = 80920 counts (through a material) ref = 139890 counts (reference beam) and the material thickness = 3.4 cm, then:80920 / 139890 = 0.5784545 (divide object counts by reference counts)
ln (0.5784545) = -0.54739539 (take natural logarithm)
-0.54739539 / 3.4 cm = -0.16099864 per cm (divide previous step by thickness in cm)
Other notes: “Virgin Teflon” can refer to any of a number of specific chemical compositions such as PTFE, FEP and PFA, among others. The exact compositions are proprietary to DuPont, the manufacturer of Teflon.
What is the atomic number, density and composition of the phantom material?
In general, the majority of our phantoms are made of Lucite ® (Polymethyl Methacrylate). This material is also known as Perspex ® and Plexiglass. Information concerning the atomic number, density and composition of Lucite ® can be found here on the web site of the National Institute of Standards and Technology (NIST).MMA has a density of 0.94 g/cm3.
The systematic name is methyl 2-methyl-2-propenoate. Its molecular formula is H2C=C(CH3)COOCH3.Information about polycarbonate can be found here, which is also a URL at NIST.
What contrast agent should I use for MRI Scans?
Experience has shown us that a 1% solution of Magnevist ® (gadopentetate dimeglumine) solution (Berlex Laboratories, Inc., Montville, NJ 07045-1000) works well for MRI imaging. Its NDC number is 50419-188-02. No doubt, there are other brands that would work equally well. To produce a 1% solution, mix 99 parts of water with 1 part Magnevist.
Why is my resolution is very poor?
You should check the following items to see if they are causing your loss of image resolution:
- inappropriate collimator
- camera is unnecessarily far away from phantom
- center of rotation (COR) correction values are not up to date
- phantom moved during scan
- poorly chosen reconstruction filter – too much blurring
- acquisition and/or reconstruction matrix size too coarse
- you may have the detector tilted with respect to the axis of rotation (this is not a possibility on some scanners)
Note: Not Having “Enough” Counts In The Reconstructed Images Does Not Affect Resolution, Except To The Extent That You Don’t Have Enough Information To See What Resolution Capability Is (Or Would Be) There With “Enough” Counts. You Essentially Have In This Case Just A Noisy, Although Possibly High Resolution, Image.
- Image Problems: I see areas that are hot, where I do not expect to see any hot area. What causes this?First make sure that you are not seeing circular artifacts caused by camera non-uniformity. If you don’t think that is the problem, look at the location of the hot area of interest. If it shows up at the exact center of the phantom, for example, it could be that you are seeing a nylon screw soaking up Tc-99m pertechnetate. The hot area in the accompanying photo below demonstrates an example of this phenomenon.
The hot area at the center of the phantom (red arrow) is actually the Tc-99m pertechnetate that has been absorbed onto the surface of the nylon hold-down screw. The center of the screw does not show any activity – yet – but in time, might eventually show activity to some degree. It has been our experience that this uptake increases over time, and may not appear if you can finish your scan soon enough after adding radioactivity to the phantom.There are several possible fixes for this problem. One way is to use a Lucite ® rod with a cap (user supplied). This will not show uptake of radioactive material onto the rod. A probably better way, which we have had good success with, is to use another radiopharmaceutical, like Tc-99m DTPA. We suspect that this phenomenon has to do with the differences in electrical charge of pertechnetate versus DTPA. Other radiopharmaceuticals might also allow for imaging without having the screw show up.Another avenue of research would be that of pre-soaking the screw with some other (non-radioactive) solution that would saturate whatever binding sites that the Tc-99m would normally stick to. If you find such a solution (pun intended), please let us know about it.
My reconstructed images are very noisy. What can you do about it?
Noisy images are generally the result of insufficient counts in the projection data, insufficient filtering, or both.
- scan for longer times
- use more radioactivity
- filter more
- add adjacent slices together
- acquire (or process) the images with smaller matrix size (thus increasing the number of counts per pixel)
- consider using “smart” reconstruction programs such as OS-EM or ML-EM instead of filtered backprojection
- I see rings in my reconstructed images. Generally, “ring” or “circular” artifacts (see accompanying image) are caused by less then adequate uniformity of camera response. This means that a uniform source of radioactivity, like a flood source, does not appear uniform in the images (projection data) acquired into the computer. A full discussion of this phenomenon can be found many other places. Usually, the cure for these artifacts is fairly simple. (Note: In the slice shown containing the rods, the cold areas between each sector and at the center of the phantom are the rods that support the spheres and the hold-down bolt, respectively, and are not artifacts.) Present day image reconstruction software usually takes care of this problem by “allowing” (translation: “forcing”) the user to acquire uniformity correction images, usually with a high number of counts – 100 million is a typical number. This image is processed to “map” the non-uniformities in the camera response and generate a correction matrix. Please see your camera’s or computer’s User Manual to determine the method of acquiring and applying flood correction images to your scans. Typically, this is necessary as often as every month to as long as every 6 – 12 months, depending on the camera and its stability.
How can I know if I really obtained the concentration (or concentration ratio) that I wanted?
If you use the methods described above for making your solutions, you will have some surplus volume. You can then obtain accurately measured volumes of sample solution and count them in an appropriate radioactivity measuring device. You might use pipettes or something like a TB syringe, depending on the volumes you want to use as samples. Depending on the number of microCuries (uCi), you could choose between counting the sample in a well counter, in a standard dose calibrator or even with your camera system.Well counters, often used for in vitro applications, are extremely sensitive and subject to overload from too much radioactivity. If you have a too high count rate from your sample at some given time and date, just wait for the samples to decay and then count them when activity levels are appropriate for the given device.In using any counting instrument, especially using dose calibrators or camera/scanner systems, you probably should take into account the background radiation. If you have calibration factors for a particular instrument for a given known number of uCi, you can relate your samples to uCi, uCi/ml, concentration ratios, etc. If you don’t already have calibration factors, you can easily determine them.To determine calibration factors, obtain a known number of uCi (using a dose calibrator) of the radionuclide you are going to measure. Then, measure this sample in your scanner (or well counter). From the number of “counts” you get in a given time frame, and accounting for decay, you can relate counts in your unknown sample back to uCi. If for example, you know that one uCi gives you 100 counts per second (using a known sample), and your unknown sample is giving you 300 counts per second, then your unknown sample is equal to 3 uCi.
Note: Because Well Counters Are So Sensitive, You Might Have To Wait Until Your Unknown Sample Decays To Below A Fraction Of A UCi To Count It To Avoid Serious Dead Time Losses. Alternatively, You Might Consider Diluting Your Sample, If That Is An Option With Your Particular Circumstances. Try Also To Make Sure That You Use As Close As Possible To The Same Counting Geometry – Same Volume, Material, Container, Placement Within The Detector Or Container, Etc.
How can I measure out such small amounts of radioactivity and volumes?
I need to make a specific concentration of radioactivity for small volumes (a few ml or less). The quick answer: Don’t! Instead of making calculations on and trying to manipulate small volumes or numbers of uCi, base your calculations on making large(r) volumes. So, if you need something like 0.8 uCi in 0.5 ml (1.6 uCi/ml), try making up 5 or 10 ml instead.To minimize radiation exposure caused by trying to get “exact” numbers of uCi, draw up some (somewhat arbitrary) larger number. Let’s say that it happens to be 76.8 uCi. To get our target concentration of 1.6 uCi/ml, we need to add the 76.8 uCi of radioactivity to a total of 48 ml of water. If you have a small volume of radioactivity, let’s say 0.1 ml, drawn up from the dose vial, then you might want to just add the 2 volumes and have a very small error in concentration:
((48.1 – 48.0) / 48.0) * 100 = 0.21 % Error
For more exacting applications, q.s. the radioactive volume drawn up from the dose vial (0.1 ml) up to 48.0 ml. In other words, add the 0.1 ml of radioactivity to 47.9 ml of water.
Is there some simple way to get desired concentration ratios of two or more radioactive solutions?
Yes! Mixing solutions to get specific concentration ratios is a real pain, especially with different total volumes! First, make up a “starter” solution. Then for your next solution of radioactivity, calculate the *same* concentration for the necessary volume and multiply the number of microCuries times your desired ratio. Then according to this total amount of radioactivity that you *actually draw up*, calculate the volume of water needed to get your desired concentration.Example: You need two solutions in a 5:1 ratio — Solution #1 (S1) needs to be 10 uCi/ml, in 200 ml
– Solution #2 (S2) needs to be 5 times that (50 uCi/ml) in 100 mlMake S1: We see that we need a total of 2000 uCi (10 uCi/ml times 200 ml). If we happen to draw up 2080 uCi, we see that we need 208 ml of non-radioactive water (2080 uCi / 208 ml = 10 uCi/ml. (We have 8 ml of spare solution: 208 ml available minus 200 needed).Make S2: Since S1 was 10 uCi/ml and we want a 5:1 ratio, that means S2 has to be 5 times S1, or 50 uCi/ml. We want S2 to have a total of 100 ml, so we need 50 uCi/ml times 100 ml, for a result of 5000 uCi.So, for making S2, we happen to draw up 5130 uCi. To get our 50 uCi/ml target concentration, we dilute 5130 uCi in 102.6 ml (which is 5130 divided by 50). Mix the 5130 uCi in the 102.6 ml and you are done! Again, the key to making this solution (no pun intended) work out is to draw up slightly more uCi of radioactivity than initially expected. That way, you can much more easily draw up “exact” volumes of water needed – much more easily than drawing up exact numbers of uCi, plus you have extra volume in case you should spill some.
Is there some simple way to get a desired concentration of radioactive solution?
If you need a specific concentration of radioactive solution, don’t draw up the specific amount of water you need and then try to get the “exact” amount of radioactivity. Instead, once you know the concentration and how much radioactivity you want, draw up a little more radioactivity than needed and then adjust the water volume. Example: Say that you want 10 microCuries (uCi) per ml, in 10 ml of water. This means you need a total of 100 uCi. It is much faster and easier to draw up a little more radioactivity than needed and then adjust the water volume. Let’s say that you happen to draw up 120 uCi. Now, to get your desired concentration, you should gather 12 ml of water (120 uCi divided by 10 uCi/ml = 12 ml).
Of course, don’t forget to take into account radioactive decay if there is significant delay between drawing up radioactive volumes from your dose vial. Also, don’t forget you might want to take into account the volume of the radioactivity that you draw up when mixing it into your stock solution. If the volume of the radioactivity you draw up is more than a few percent of that of the “cold” water you are going to mix it with, you might wind up with significant errors in concentration.
This method allows you to handle radioactive material once or twice at most, and instead can spend more time accurately gathering the exact volume of non-radioactive water.
Yes, you will have 2 ml of excess volume, which is actually desirable. If you have *exactly* the volume you need and should spill some, then you will have to either make up the volume with non-radioactive water which would change your target concentration (or live with an air bubble), or you would have to make up another batch.
Again, since you are dealing with radioactivity, you want to minimize the amount of time spent handling it.
One more (repeated) reminder: Especially when dealing with small total volumes, do not forget to take into account the volume of radioactivity solution you draw up from the dose vial! It could cause an unintended error in your total volume leading to an error in desired concentration.
For example, if you withdraw 100 uCi from the dose vial in 0.2 ml , and you want a *total* of 1.0 ml, be sure to add your radioactivity to 0.8 ml. Otherwise your concentration will be: 83.3 uCi/ml = 100 uCi / 1.2 ml instead of 100.0 uCi/ml = 100 uCi / 1.0 ml for an error of about 17%.
The key to making this solution work out correctly is to draw up slightly more uCi of radioactivity than initially expected. Then, you can easily draw up “exact” volumes of water needed – much more easily than drawing up exact numbers of uCi. Additionally, you have extra volume in case you should spill some.
In summary:
- Determine your desired concentration and desired water volume.
- From that information, calculate the necessary number of uCi.
- Draw up a little more radioactivity than needed.
- Based on the number of uCi, calculate the new volume of water now necessary to obtain your desired concentration.
- When dealing with small volumes (relative to the volumes of radioactivity withdrawn from your dose vial), be sure to take into account the volume you draw up when adding that to your non-radioactive volume of water.
How can I measure the amount of water that I've added to each sphere?
There are several approaches to determining the amount of water added to the spheres. For these smaller spheres you can either try to determine the amount from directly reading before and after filling syringe volumes. If you use this approach, here are some hints for relatively accurate measuring:
A. Use a TB type syringe, whose total volume is 1 ml or less.
B. Be certain to get all bubbles out of the syringe, including the needle.
C. Before filling the sphere, squirt out 1 or 2 drops of water into a waste receptacle; this helps ensure no air was left in the needle. Write down the volume shown on the side of the syringe.
D. Subtract the post-filling amount of syringe contents from the pre-filling amount to calculate the water volume inserted into the sphere. The other method involves the weighing of the sphere before and after filling, calculating the weight, and converting to volume. The weights will be on the order of grams, and so you will need a fairly sensitive device.
One that works well for this application is the Mettler PE 360 (Mettler-Toledo, Inc., 1900 Polaris Parkway Columbus, OH, 43240, Phone: +1 614 438 4511). This method assumes that a gram of water corresponds to 1 ml of volume. Weigh the sphere before and after filling, and subtract the weights to determine the water weight. Convert grams to ml to get the volume. Example: A sphere weighs 20.0 and 25.1 grams before and after filling, respectively. The weight of the water is 5.1 grams, and thus the volume is 5.1 mls. Note that this assumption may not hold true for solutions of water and other components, like CT contrast media. In this case, you can determine the weight of the solution (put a known number of mls in a container that you have weighed before and after putting the solution in) and then determine ml per gram. Unknown volumes can be determined by calculations based on the weight of the test solution. Example: A 5 ml solution weighs 5.8 grams. So the solution is 1.16 grams/ml. Now if the sample whose volume is unknown has a weight of 2.32 grams, we divide 2.32 by 1.16, for a volume of 2.0 ml. Note that these are just some “made up” example numbers and may not be similar to your solutions.Note that sensitive scales are, well, sensitive. This means that you should use the shields that come with the scales to seal the measuring chamber against air currents that will produce erroneous measurements. Other tips to using (electronic) scales:
A. Allow sufficient time for the instrument to warm up after turning it on. The various components will drift over time until they stabilize, which might take 15-30 minutes or so.
B. Make certain that the scales are on a flat, level surface. “Level” the adjustable feet on the instrument, if it has this type. Some instruments have a built-in bubble level.
C. Some scales have “tare” capability. This means that you can, after allowing the machine to warm up and stabilize, place the empty sphere on the scales and “zero” the reading on the display. After you fill the sphere and weigh it again, you can then read the weight of the water in the sphere directly off the display without having to perform any arithmetic calculations.
D. Avoid making the measurements if the scales are placed in a location that experiences a lot of vibrations on the table-top. These can come from HVAC systems, large passing trucks, nearby construction, etc.
How do I care for and clean my phantom?
We recommend that you occasionally clean the phantom to remove any water residue build-up. For this purpose use only mild soap solutions. NEVER use chlorinated hydrocarbons (e.g., methylene chloride, ethylene dichloride, methyl ethyl ketone, etc…), alcohol, kerosene or benzene to clean the phantom since these could etch, or even dissolve, the acrylic (polymethyl methacrylate (PMMA)) or could erode the bonds holding the phantom together. If the phantom is not to be used for a few weeks, a very low concentration of chlorine and water solution of less than five (5) parts per million (as determined with a pool chemical test kit, for example) may be used to retard bacterial or algae growth within the phantom. However, we recommend that the phantom be completely drained and dried if it is not to be reused in the near future. DO NOT use any mechanical tools when tightening filler plugs. HAND TIGHTEN the filler plugs, otherwise you may damage the phantom if using pliers or other tools. While the delrin or nylon filler plugs are tough, they can still be damaged by misuse. We recommend that you do not over-tighten the filler plugs. Never replace nylon or “plastic” parts such as screws with metal equivalents. Nylon screws and bolts have been provided so that they will “give” when a certain amount of pressure has been applied to them. Generally, it is much preferred that if something has to break, it should be inexpensive parts, such as screws, rather than the phantom itself. Metal replacement parts could damage or destroy your phantom, so if you need replacements, please be sure to use parts equivalent to the original ones. Apply small amounts of silicone grease or a commercially available O-ring grease when the O-rings feel or appear “dry”. Silicone grease is available in the plumbing department of home improvement stores, such as Lowes or Home Depot. Though it might seem obvious, we remind you that the phantom should not be dropped on the floor. Its size and shape might lend itself to being accidentally dropped, but bear in mind that the Limited Warranty will be voided if Data Spectrum Corporation determines that the phantom has been physically damaged by the user. Handle the phantom and inserts with care. Each component is precision and custom-made. Avoid dropping or striking since acrylic is brittle and relatively fragile.
How do I know what the SPECT resolution of my system should be?
First, make a capillary line source and mount it in front of the camera at some known distance, let’s say 15 cm, from the front surface of the collimator. Make sure that it is lined up “straight” (parallel) with the edges of the acquisition matrix, so that its long axis is “vertical” in the computer image. Then acquire a planar image of it, and draw several “horizontal” profiles through the image of the line source. Make the profiles several pixels thick. From these profiles, determine a typical or average FWHM and FWTM of the planar image.Now, perform a SPECT scan of the line source, with the source mounted as close as possible to the axis of rotation. Use the same matrix size and zoom as in the planar image. The radius of rotation should be set to the same value used as the distance in the planar image. In this example, it was 15 cm.Once you reconstruct the images, your transverse slices will contain “dots”, instead of a line. Remember: you are making transverse cuts, or slices, of the line and are now looking at the line from a different perspective. Now draw profiles (using the same profile thickness as in the planar images) that encompass the “dot” of activity. (It doesn’t matter if you use vertical or horizontal profiles in this image.) Analyze the FWHM and FWTM from the profiles in the transverse image. Now, compare the FWHM and FWTM values taken from the planar image with the values taken from the SPECT image. For typical filtered backprojection programs and a ramp filter, you should expect the SPECT FWHM values to be no more than approximately 10% worse than the planar images. Reasonable and approximate line source parameters:
- relatively fine acquisition matrix and pixel size (use the smallest pixel size your system supports if you want to determine “ultimate” resolution) – 2mm or less should be sufficient
- 120 to 180 angles for SPECT scan, over a 360 deg. range
- line source approximately 5 to 10 cm long, containing 2 to 10 milliCuries
- collimator: user selected
- planar image acquired for 1 to 3 minutes; SPECT scan acquired for total of about 3 to 10 minutes; be sure not to allow pixel overflow in planar or SPECT acquisition, and in the reconstruction; adjust time accordingly
- How do I make my own capillary line sources and how do I fill them? You can order bulk amounts of glass capillary tubing from scientific supply houses. One example is Kimble #46485. Tubes should be about 1mm inside diameter for clinical-type scanners. Once you have your tubing, you can cut it to the desired length with a small, metal triangular file. This is a technique that takes only a small amount of practice to become proficient at. Using very light pressure, hold the tube in between your thumb and forefinger and gently press one corner of the file against the tube where you would like to cut it. (Holding the tube in the fingers works better for the author of this FAQ, rather than trying to lay the tube on a hard surface.) Using too little pressure will fail to scratch a mark on the glass, while too much pressure will basically shatter the tube at that mark. So, take care not to cut yourself; We suggest you don protective eyewear. Once you have a scratch mark at the desired location, put the thumb and forefinger of one hand on one side of the scratch mark and close to it, then do the same for the other hand on the other side of the scratch. Have the scratch mark away from you. Gently bend the tube and it should break cleanly, with a nice even edge on each new end of tubing. Filling the tubes also requires requires a small amount of practice, which you can do with non-radioactive water. The tube may be filled using capillary action by tilting one end of the tube and touching it to the surface of a drop of the radiotracer solution. By holding the other end closed, the tube may then be removed from the solution and sealed with a sealer such as “SealEase” or with a putty compound such as “Mortite”. The author normally seals both ends. This way, if the source should break, there is a likelihood of less solution leaking out the unsealed end of tubing. After filling and sealing your line source, you might want to rinse the end touched to the radioactivity and dry the line source. Of course, please take appropriate precautions not to accidentally break the source, as you could cause radioactive contamination of other objects.
How can I measure the resolution of my system?
You can estimate resolution somewhat accurately by looking at reconstructed images of certain phantoms/inserts (cold rods, for example). By knowing the rod diameters for given sectors of the rod insert, you look to see if most rods in that sector are visible. You can then say that resolution is approximately whatever the rod sizes are …… but that does not answer your question. To actually measure your resolution you should perform a scan of something like capillary line sources. The DSC QC phantom (ECT/FL-DLX/P) is designed so that (typically glass) capillary line sources can be mounted in the filler caps of the phantom. You can also devise your own method for mounting sources and do not need a special phantom to perform this measurement, other than the tubes themselves. Please see the Users Manual for how to actually mount them in the QC phantom. Information is available on how to make your own and where to buy glass capillary tubes. Once you have scanned and reconstructed the line sources, you can use your computer’s software to get image profiles. If you are lucky, your computer will have a quick method of determining the full-width at half maximum (FWHM) and full-width at tenth maximum (FWTM) from the profiles. If not, you hopefully can print out the profile values and input them into third party software that can perform these calculations.
What kind of water do I use to fill the phantom?
A frequently asked question is that of what kind of water to use. Users may have access to demineralized, deionized, distilled, bottled water from grocery stores or other commercial suppliers, or tap water. Generally Data Spectrum suggests that you use bottled or tap water, as long as the tap water does not contain high amounts of iron, lime or other minerals. Such minerals might deposit themselves on the inner walls of the phantom or in theory combine with the radiopharmaceutical you use. This could, in theory, change the distribution of radioactivity. Bottled water is generally readily available, so it is a good choice to start with, as is low-mineral content tap water.Internal discussions within Data Spectrum Corporation with consultants have led to the supposition that distilled and/or deionized water might have chemical characteristics that would allow for reactions with some radio-pharmaceuticals. In theory, this might result in surface absorption of radioactivity on (nylon) bolts or some other non-uniform distribution of the radioactivity. Several years ago, a customer (a physicist) of DSC stated that he he had put deionized water into a phantom, filled it with Tc-99m and imaged it. He then drained the phantom and found that the cold rods had “absorbed Tc-99m on the surface somehow and the rods were radioactive. DSC employees duplicated this by using distilled water. The theory of DSC at the time was that Tc-99m pertechnetate is charged (ionic) and because distilled water and the PMMA rods are both good insulators the Tc-99m ions were somehow attracted to the PPMA rods. DSC found that the problem went away when tap water was used. DSC did not investigate this further. It is possible that if we had added some kind of salt (say NaCl or CaCl or something else) to the distilled water, the problem would not have occurred. DSC suggests that customers determine if their deionized and/or demineralized water causes this problem should they observe unexpected hot areas in their reconstructed images.
What if the phantom leaks?
The phantoms are designed so that with minimal effort, they will not leak. Generally, a leak is going to occur when O-rings are either missing, improperly seated in their groove, or if it is dried out. Check these considerations first if your phantom leaks. You can try to use some silicone lubricant from the packets provided with your phantom to help create a better seal. Beyond that, Data Spectrum recommends you call us at (919) -732-6800 or email info@spect.com to make a phantom check-up appointment. The RMA (Return Materials Authorization) will allow Data Spectrum professionals to assess your phantom and quote what needs to be replaced or repaired. The phantom will go through rigorous testing before leaving the shop just as it did when it was new and you’ll be worry free for many scanning sessions.
What if I break or lose a part of my phantom?
How do I get replacement parts? Replacement parts are available from DSC. Please contact us if you want to order parts. You can set your institution up for a routine shipment of new o-rings, screws, etc by calling us or emailing us at sales@spect.com. This will ensure you have what you need when you need.
What is the warranty on DSC Phantoms?
This is our “standard” warranty (please see your Users Manual for the specific phantom you are inquiring about):
Data Spectrum Corporation warrants the product to be free from defects in materials and workmanship, under normal use, for a period of ninety (90) days upon receipt of product(s). If Data Spectrum Corporation receives notice of defects in materials or workmanship during the warranty period, Data Spectrum Corporation will either, at its option, repair or replace product(s) that proves to be defective. With continued responsible and careful use, the product(s) should provide excellent service. Exclusion: The above warranty shall not apply to defects resulting from improper or inadequate use or maintenance by the buyer (or representative thereof), unauthorized modification or misuse, operation outside of the environmental specifications of the product, improper site preparation and maintenance. Data Spectrum Corporation or any authorized representative will not be liable for any expenses incurred outside this warranty. Data Spectrum Corporation does not warrant that the functions contained in the product(s) or manual(s) will meet each individual requirement, or that the operation of the product(s) or manual(s) will be uninterrupted or error free. Warranty Limitations: Data Spectrum Corporation makes no other warranty, either expressed or implied, with respect to this product(s). Some states or provinces do not allow the exclusion of implied warranties, so the above exclusion may not apply. This warranty gives the buyer specific legal rights, and the buyer may also have other rights that vary from state to state.
What should my quality control scan consist of?
Generally, once a month, you should scan your QC phantom with these approximate parameters, depending on your specific site configuration and your needs:
- high resolution or general purpose collimator
- 30-60 milliCuries of Tc-99m (SPECT scanners)
- 128×128 acquisition matrix, zoom 2.0 (or the equivalent of about 1.7 mm/pixel)
- 120 angles (“stops”) per camera, with a full 360 deg. rotation of head(s)
- scan for about 30-60 seconds per angle
- reconstruct with desired filter (start with ramp filter), attenuation compensation (either measured w/TCT (Transmission Computed Tomography) scan, or assume 0.12 per cm); add anywhere from 10 to 50 slices in the rods section together; add 3 to 4 slices of spheres section together; add 10 to 20 slices of uniform section together
- for multi-camera scanners, reconstruct each head individually and then all heads combined
- inspect for presence of artifacts, resolution, etc.
- compare with previous scans; archive results
We are told that some institutions set up their QC scan to begin as the technologists are leaving work, allowing the scan to take place after normal working hours, and then processing it the next day. You should first determine that the use of this method complies your institution’s regulations and state and federal regulations with regards to unattended scanner operation, and with regards to concerns of possible unauthorized access to radioactive objects.
How often should I perform my quality control procedure?
Generally speaking, one should perform a Quality Control scan about once per month. You might consider performing your QC scan when:
- installing a new system
- after having “major” retuning or repairs on the scanner
- after new upgrades of software
- “certifying” or training new personnel
How do I reconstruct my scan images?
Generally, you should use the same matrix size, or finer, for reconstruction as was used for the acquisition (if you have a choice), and you should initially start with the least amount of filtering. Typically, this can be a ramp filter for filtered back projection reconstruction programs (the most widely available type). Keep in mind that you will have the most noisy images with a ramp filter, but if you are looking for resolution, the ramp filter will give you the least amount of blurring of the image. Remember, you are not looking for “pretty” images – you are looking for presence of artifacts or fine structures (the rods or spheres) in the phantom – and these can disappear if you use too low of a cutoff frequency in your filter.
Quick Filter Lesson: Filters Can Be Generally Thought Of As Blurring Functions, That Is, They Only Allow Lower Spatial Frequencies To Be Used During The Reconstruction Process. Thus, They Are Often Called “Low-Pass” Filters. Filters Are Typically Designed With At Least One (But Sometimes More) User Specified Parameter Known As The Cutoff Frequency. This Value Is Usually Expressed In Cycles Per Cm, But Can Be In Other Units. A Larger Cycles/Cm Value Means That Less Blurring Is Taking Place. Thus, A Filter With A Cutoff Frequency Of 1.6 Cycles/Cm Does Less Blurring (And Looks Noisier) Than One With A Value Of 0.5 Cycles/Cm.
Reconstruct each head individually, and then all heads combined. Perform attenuation compensation with either a measured TCT (Transmission Computed Tomography) map, or use a value of 0.12 per cm constant value.
Note: The True Attenuation Coefficient Values For Water And Lucite ® Are Approximately 0.15 Per Cm At Tc-99m Energies. Using A Value Of 0.12 Instead Of The True Value Will Artificially “Compensate” For The Extra Apparent Activity Near The Center Of The Cylinder That Results From Scattered Photons. Note That This Is An Ad Hoc Method, And That It Provides A Close Enough Approximation For Many Applications. It Will Result In A Relatively Flat Profile Across The Transverse Images.
Another Point To Remain Aware Of Is That The Reconstruction Software Of Some Scanner Systems Is Configured To Use Units In Terms Of Cycles Per Pixel Instead Of Cycles Per Cm. You Should Determine If Your System Is This Type And Make Adjustments Accordingly. Contact The Vendor Of That System To Obtain Values That Are Appropriate For Your Uses Of The System. It Is Impossible For Us To Provide A Specific Value, Since Converting From Inverse Centimeters To Inverse Pixels Would Depend On The Matrix Size And The Field Of View Of The Camera.
For the cold rods sections of the QC phantom, you probably will want to add 10, 20 or more slices together to improve rod visibility and reduce noise. For this to be successful, you must position the phantom in the scanner as perfectly aligned with the axis of rotation as is possible. Otherwise, any tilting will mean rod locations in one slice will be slightly shifted in subsequent slices. When you add them, the rods will not “line up” and you will just have blurred images.
For the slices encompassing the spheres, pick the 2 to 4 slices that seem to include the smallest sphere, and then add these images together. In the uniform section of the cylinder (the volume between the spheres and the phantom’s cover plate), add together 10 to 20 or so of these slices.
Note: Depending On Your Computer’s Software And The Number Of Counts In The Transverse Images, You Might Have To Scale The Images Down Before Adding Them, So That You Don’t Have Overflowed Values In Some Pixels. Basically, This Just Means You Have To Divide The Images By Some Factor Like 2 Or 5 Or 10 Before Adding Them. Typically, Most Nuclear Medicine Images Today Are Arrays Of 2-Byte Integers (The “Pixels”), Which Means That The Pixel Can Store A Value No Higher Than 32767. (There Is At Least One System On The Market That Has 2 Byte Integers That Have A Value Range From 0 To 65K, But I Did Say “Typically”, Above). If A Pixel Is Told To Be Set To Some Number Greater Than 32767, It “Overflows”. The Value Is Too High For The Integer Format Declared In The Program’s Source Code, And May (Internally In The Particular Bit Pattern Of The Bytes) Appear To Be Negative Or Some Astronomically High Number. It Will Depend On The “Sign” Bit And The Variable’s Declaration In Source Code.
Do you have any helpful hints and precautions for filling/draining my phantom?
- Wear gloves, lab coat and protective eye-wear when dealing with radioactivity.
- Use time, distance and shielding as much as is practical to reduce radiation doses to personnel.
- Following a well thought out plan of action will avoid wasting time and will help reduce radiation exposure. Ask yourself “Exactly what is it I want to learn from this scan?”. This will help make sure that you will obtain the desired information and it will help reduce the number of repeat scans later on that you will have to perform.
- Have all your materials (tools, phantoms, mixing containers, etc.) ready before beginning your experiment. This will help reduce the amount of time you are exposed to radiation
- Fill the phantom with water that is at or slightly above room temperature (but not hot!). This will help to:
- prevent the potential cracking of the phantom when the water volume increases as water temperature equilibrates with room temperature (if you were to start with cold water). Since the sealing ability of the caps (with O-rings) is very high, increased pressure in the phantom might in rare instances damage the phantom.
- prevent water from spraying out when a cap is removed. While the number of ml’s involved would be very small, we wish to alert you to the potential for contamination.
- create a very slight vacuum as the water cools, which will help insure against leakage if the caps or O-rings are not properly sealed. Normally, leakage would be a rare event with ordinarily encountered temperatures.
How much radioactivity do I put in the phantom?
For most QC scans with general purpose or high resolution collimators, you should start with about 30 to 60 milliCuries. Adjust either your scan time or activity levels after that, depending on what result you obtain and what result you would like to obtain. For more complex scans, you may want to contact your camera manufacturer or the Physics, Instrumentation and Data Sciences Council through the Society of Nuclear Medicine.
How do I position the phantom?
Again, this question is broad enough that we can only refer you to your Users Manual you received when you purchased your phantom. There are so many phantoms, applications and scanners that it would be nearly impossible to answer in just a few short sentences. Generally speaking, you will want the long axis of cylindrical type phantoms parallel to the axis of rotation. You will want the phantom centered in the scanner’s field of view (this is not so important for MRI scanners). The reason for this is so that you can move the camera head(s) as close as possible to the phantom during the scan.
This will obtain better resolution than having the camera(s) far away from the phantom. You will have to determine how you actually “mount” or suspend the phantom in the scanner opening. Many people lay the phantom on a blanket or similar object on top of their scanner’s patient bed.
Other institutions have made special holders that clamp onto or replace the head holder. Others have used the collimator changing cart as a support, with adapters to actually support the phantom. Once you have decided upon a mounting method, and while monitoring the phantom position on the persistence scope or computer monitor, the angulations and tilts of the phantom can be adjusted prior to the scan.
While still in “persistence” mode, rotate the camera to top, bottom and sides of the phantom. Adjust the phantom position based on these images. Generally, you will look for the edges of the phantom to be parallel, or “square”, with the edges of the P-scope.
How do I fill my phantom?
First, read through your Users Manual that came with the phantom that you own. There are so many different phantoms that no single filling guide is going to cover the details you might want to know for every one of DSC’s phantoms. However, here are some general pointers to simplify filling your phantom.In general, you probably will have the easiest time of filling your phantom by observing these guidelines:
- First and foremost, decide on what it is that you want to do! Know ahead of time what it is you want to learn from the scan. Decide before the planned scan time what concentration ratios (if applicable), acquisition parameters, scan length, radioactivity levels, etc. that you want to use. If your experiment depends on concentration ratios, measure the volumes of the chambers that you are going to fill.
- Remember: You are dealing with radioactive materials, so you want to minimize the amount of “fiddling around” measuring volumes, deciding on phantom positioning, total amount of radioactivity to use, etc. You might want to re-read the previous guideline in this series.
- If possible, get together the water to fill the phantom with several hours, or even a day, ahead of preparation time. This will allow dissolved air to escape out of the water, leading to fewer bubbles forming during your scan, and will allow it to equilibrate to room temperature.
- Pre-mix the radioactive tracer with water in external containers instead of injecting the radioactivity into an already filled phantom. This is usually always easier and provides a more uniform solution.
Contact Us
If you would like to learn more about our products or want to talk with someone about the use of our Phantom products to obtain ACR accreditation feel free to call us at
(919) 732-6800
or complete our contact form.
Click here for questions regarding ACR accreditation.